If you’re looking for a more affordable alternative to Norvasc, the Norvasc formulary generic equivalent could be the answer you need. These generic medications contain the same active ingredient as the brand-name Norvasc, providing the same therapeutic benefits but at a fraction of the cost.
Switching to a generic version of Norvasc can significantly reduce your out-of-pocket expenses while ensuring you receive the same high-quality treatment. Generics are held to the same strict safety and efficacy standards as their brand-name counterparts, making them a reliable and cost-effective choice for managing your condition.
To explore your options, consult with your healthcare provider about the Norvasc formulary generic equivalent that may be suitable for your needs. They can provide guidance on the appropriate dosage and assist you in navigating the transition to a generic medication.
- Norvasc Formulary Generic Equivalent
- Comparing Norvasc and Generic Amlodipine
- Cost Savings with Generic Amlodipine
- Identifying Generic Equivalents of Norvasc
- Comparing Costs: Norvasc vs. Generics
- Ensuring Bioequivalence and Efficacy
- Regulatory Compliance
- Clinical Outcomes
- Consulting Your Doctor and Pharmacist
Norvasc Formulary Generic Equivalent
Consider switching to a generic equivalent of Norvasc (amlodipine) if it is prescribed for you. Generic amlodipine is typically more cost-effective than the branded Norvasc, while providing the same active ingredient and therapeutic benefits. Speak with your healthcare provider to determine if a generic amlodipine is suitable for your condition and treatment plan.
Comparing Norvasc and Generic Amlodipine
The active ingredient in both Norvasc and generic amlodipine is amlodipine. Generic versions are required to meet the same quality, safety, and efficacy standards as the brand-name drug. They contain the same amount of the active ingredient and work the same way in the body.
Cost Savings with Generic Amlodipine
Generic medications typically cost significantly less than their brand-name counterparts. Switching to generic amlodipine can provide substantial cost savings, especially for those paying out-of-pocket or with high deductible health plans. Consult your pharmacist or insurance provider to understand your potential savings.
Identifying Generic Equivalents of Norvasc
Check your prescription label. The generic name for Norvasc is amlodipine. Look for “amlodipine besylate” on the generic medication label. This is the active ingredient you need to match.
Consult your pharmacist. They possess comprehensive knowledge of available generics and can directly compare various brands to ensure equivalence in terms of dosage form and bioavailability. They can answer any questions you may have about specific products.
Use a reliable drug database. Websites like the FDA’s DailyMed or similar reputable sources provide detailed drug information, including a list of approved generic equivalents. Carefully compare the listed information to your prescription.
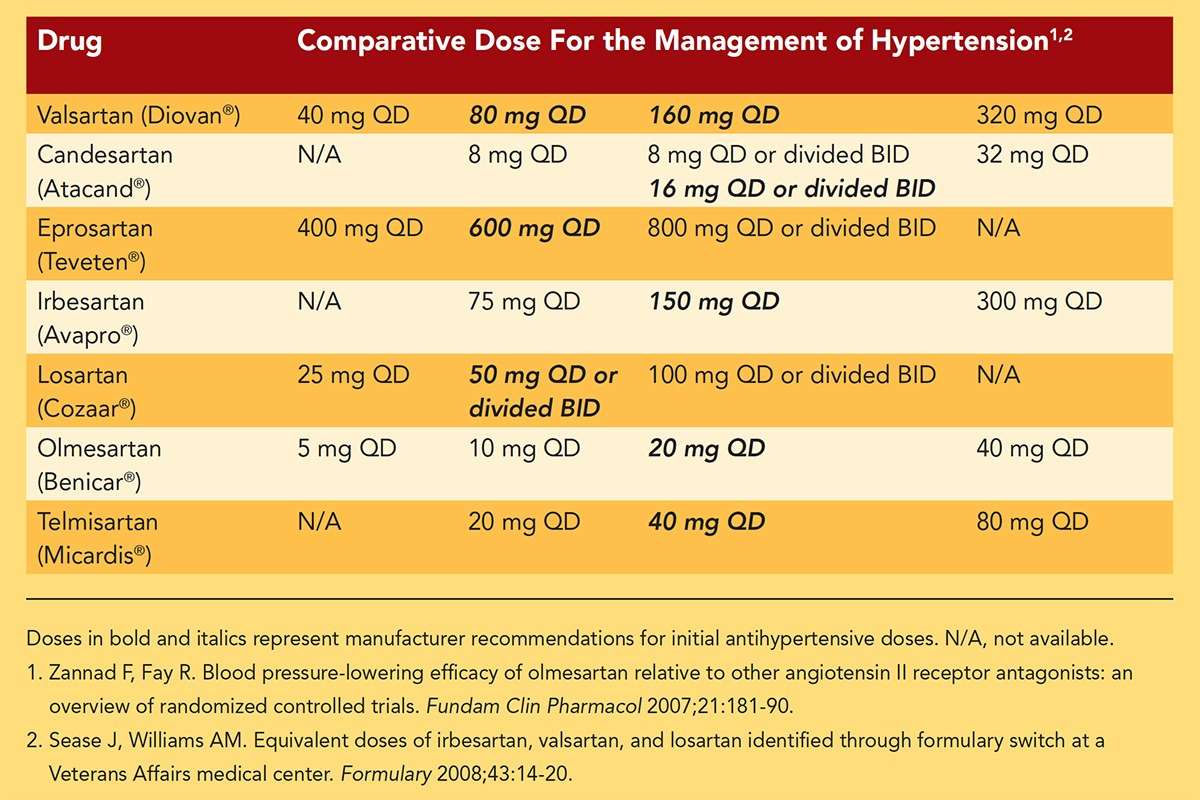
Compare the dosage. Ensure the generic medication matches your Norvasc prescription in strength (mg) and dosage form (tablet, extended-release tablet, etc.). A discrepancy here could significantly impact treatment.
| Factor | Check for Match |
|---|---|
| Active Ingredient | Amlodipine Besylate |
| Dosage Strength | Match your Norvasc prescription exactly (e.g., 5 mg, 10 mg) |
| Dosage Form | Tablet, Extended-Release Tablet, etc. – must be the same |
| Manufacturer | Note the manufacturer for reference (though not the sole determinant of equivalence) |
Contact your doctor. If you have any concerns or uncertainties about a specific generic, or experience any unexpected side effects, consult your physician immediately. This is the best way to ensure safe and effective treatment.
Comparing Costs: Norvasc vs. Generics
Generic amlodipine, the active ingredient in Norvasc, consistently costs significantly less. Expect to pay a fraction of the brand-name price.
Price variations exist. Your out-of-pocket expense depends on your insurance coverage and the pharmacy. Use a prescription drug pricing website or your insurance’s website to compare prices before filling your prescription. Many pharmacies offer discount programs.
Consider using a drug discount card. These cards can offer additional savings on both brand-name and generic medications. Check for eligibility requirements.
Manufacturer coupons for Norvasc may occasionally lower the brand-name cost. However, the generic will usually remain cheaper.
Always check with your doctor before switching between brand-name and generic medications, although amlodipine generics are generally considered bioequivalent to Norvasc.
Savings are substantial. By choosing the generic version, you can save considerable money on your medication without compromising efficacy.
Ensuring Bioequivalence and Efficacy
To ensure bioequivalence and efficacy, it is crucial to adhere to rigorous regulatory guidelines. Firstly, the generic formulation must demonstrate comparable pharmacokinetic (PK) parameters, such as peak concentration (Cmax) and area under the curve (AUC), to the reference listed drug (RLD). This is typically achieved through well-designed bioavailability studies involving healthy volunteers.
Regulatory Compliance
Manufacturers must submit comprehensive data to regulatory authorities, demonstrating that the generic product meets the required bioequivalence criteria. This includes providing information on the drug’s formulation, manufacturing process, and quality control measures. Regulatory bodies, such as the FDA, closely review this data to ensure that the generic drug is therapeutically equivalent to the RLD.
Clinical Outcomes
In addition to bioequivalence, it is essential to assess the clinical efficacy and safety of the generic product. Post-marketing surveillance and real-world evidence studies can help evaluate the effectiveness of the generic drug in the target patient population. Monitoring adverse events and patient-reported outcomes can further validate the therapeutic equivalence of the generic formulation.
- Conduct rigorous bioavailability studies to establish bioequivalence.
- Comply with regulatory guidelines and provide comprehensive data to regulatory authorities.
- Evaluate clinical outcomes through post-marketing surveillance and real-world evidence studies.
- Monitor adverse events and patient-reported outcomes to ensure therapeutic equivalence.
By adhering to these principles, manufacturers can ensure that their generic formulations of Norvasc (amlodipine) are both bioequivalent and clinically effective, providing patients with a safe and cost-effective alternative to the branded medication.
Consulting Your Doctor and Pharmacist
Discuss any concerns you have about Norvasc or its generic equivalent with your doctor. They can provide personalized guidance on your medication and address any questions or issues you may have. Be sure to mention if you have any other medical conditions or are taking other medications, as these factors can influence the appropriate treatment plan.
Additionally, consult your pharmacist when filling your prescription. They can provide valuable information about the medication, including dosage instructions, potential side effects, and any interactions with other drugs you may be taking. Pharmacists are a great resource for ensuring safe and effective use of your medication.
Remember, open communication with your healthcare team is essential for getting the most out of your treatment. Don’t hesitate to ask for clarification or express any preferences you have regarding your medication. Working together, you can find the best solution to manage your condition.