Generic versions of Viagra are expected to hit the market soon, providing a more affordable option for those seeking treatment for erectile dysfunction. Current estimates suggest that the FDA is likely to approve generic formulations within the next one to two years. This timeframe aligns with patent expirations and ongoing regulatory reviews.
Manufacturers are already gearing up to meet the anticipated demand. Several pharmaceutical companies are conducting trials and preparing their applications for generic sildenafil, the active ingredient in Viagra. Once approved, consumers will benefit from significant cost reductions compared to brand-name products.
In the meantime, individuals interested in generic Viagra should consult with healthcare providers to explore available alternatives. Discussing both branded and potential generic options can help tailor a treatment plan that aligns with budgetary needs and health considerations. Stay informed on industry updates to take advantage of the opportunities that generic Viagra will present.
- When Will Generic Viagra Be Available
- Current Patent Status of Viagra
- FDA Approval Process for Generic Drugs
- Timeline for Generic Viagra Release
- Patent Expiration and Generic Market Entry
- Market Dynamics and Consumer Access
- Factors Influencing Market Availability
- Regulatory Approval
- Market Dynamics
- Impact of Generic Viagra on Pricing and Accessibility
- Pricing Dynamics
- Accessibility Improvements
When Will Generic Viagra Be Available
Generic Viagra is anticipated to become available in the United States from 2025 onwards. This timeline follows the expiration of the patent for the brand-name drug. Various pharmaceutical companies are preparing to launch their generic versions, which will increase accessibility and affordability for patients.
As the market evolves, generic manufacturers will likely seek to deliver competitive pricing. This shift will open up new opportunities for consumers to obtain erectile dysfunction treatments without the financial burden associated with branded options.
Engagement with healthcare providers remains essential. Doctors can provide guidance on the transition to generic versions, addressing any concerns about efficacy and safety. This communication ensures patients receive the right dosage and understand potential side effects.
Below is a table outlining key dates regarding the availability of Generic Viagra:
| Event | Date |
|---|---|
| Patent Expiration | 2025 |
| Potential Launch of Generic Versions | 2025 onwards |
Keep an eye on news from pharmaceutical companies for updates on product releases. Early adoption of generic drugs can lead to significant cost savings, allowing more individuals to seek necessary treatment.
Current Patent Status of Viagra
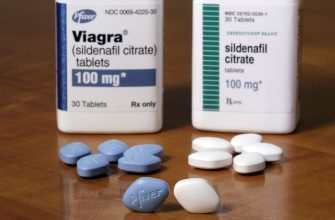
Viagra’s patent, originally filed in 1996, expired in April 2020. This opened the door for generic versions to enter the market. The active ingredient, sildenafil citrate, can now be produced by various manufacturers, allowing for a more affordable option for patients.
Since the expiration, multiple pharmaceutical companies have sought to develop and market generic formulations. The approval processes by the FDA have accelerated, enabling these generics to become widely available. Patients should check with their pharmacies for options, as generic sildenafil offers similar efficacy and safety profiles as the brand-name product.
Continued patent protections still exist for some related formulations and delivery methods, such as the oral spray. However, these do not affect the availability of generic sildenafil. Always consult a healthcare provider for recommendations on choosing between brand-name and generic medications.
FDA Approval Process for Generic Drugs
The approval of generic drugs by the FDA follows a set protocol to ensure safety and effectiveness. Initially, a manufacturer must submit an Abbreviated New Drug Application (ANDA). This application demonstrates that the generic drug is equivalent to the brand-name counterpart in dosage form, strength, route of administration, and intended use.
During the review, the FDA evaluates the generic’s formulation and ensures that it meets the same quality standards as the brand-name drug. Clinical studies are often required to confirm bioequivalence, which shows that the generic produces similar therapeutic effects in patients. The FDA also inspects manufacturing facilities to guarantee compliance with good manufacturing practices.
The FDA prioritizes applications based on various factors, such as the potential clinical impact of the drug and the public health need. Upon successful review, the FDA grants approval, allowing the generic drug to be marketed. Manufacturers must continue to meet rigorous quality controls and report any adverse effects post-marketing.
In summary, the FDA’s stringent approval process for generic drugs ensures that these alternatives are both safe and effective, allowing patients access to affordable treatment options.
Timeline for Generic Viagra Release
The availability of generic Viagra is anticipated near the middle of 2025, following the conclusion of the original patent protections. This timeline is aligned with ongoing developments in pharmaceutical regulations and market approvals.
Patent Expiration and Generic Market Entry
Viagra, known for its active ingredient sildenafil, has been under patent protection for many years. The original patent expired in April 2020, but exclusivity agreements delayed the entry of generics into the market. As these agreements lapse, generic manufacturers are preparing to launch their products, likely starting in early 2025.
Market Dynamics and Consumer Access
Once generics are approved, consumers can expect significant price reductions compared to branded versions. The competition among various manufacturers will further accelerate price drops, enhancing accessibility. Pharmacies will stock generic options quickly, ensuring easy access for those in need.
Monitoring announcements from pharmaceutical companies regarding their plans to launch generic versions will provide the latest updates on this timeline.
Factors Influencing Market Availability
The market availability of generic Viagra is affected by several key factors. Patent expiration plays a primary role; once the patent for the original drug expires, other manufacturers can produce and sell their versions. This usually paves the way for competitive pricing and increased accessibility.
Regulatory Approval
Regulatory approval is another crucial aspect. Generic manufacturers must navigate through rigorous evaluation by agencies such as the FDA in the United States. This process ensures that the generic version matches the original in potency, safety, and dosage form. The timeline for approval can impact how quickly generics hit the market.
Market Dynamics
Market dynamics, including supply chain logistics and manufacturing capabilities, also influence availability. Manufacturers must establish reliable production methods and distribution channels to meet consumer demand. Economic factors, such as pricing strategies and competition among manufacturers, can further shape the market landscape, affecting how soon the public can access generic versions.
Impact of Generic Viagra on Pricing and Accessibility
Generic Viagra significantly reduces the cost for consumers, making treatment more accessible. With the entry of generics into the market, prices typically drop by 50% to 80%. This allows more individuals to afford their prescriptions without compromising their health or budget.
Pricing Dynamics
Manufacturers of generic medications often face lower production costs, leading to competitive pricing. Key factors influencing this include:
- Reduced marketing expenses compared to brand-name drugs.
- Increased competition among multiple generic producers.
- Availability of the active ingredient without extensive research and development costs.
As a result, pharmacies may offer generic Viagra at lower prices, providing multiple purchasing options for consumers.
Accessibility Improvements
With the introduction of generic Viagra, access to treatment improves for various demographics. Some benefits include:
- Expanded reach to uninsured or underinsured individuals.
- Easier procurement through online pharmacies, often at lower prices.
- Pharmacy benefit managers may include generics on formularies, increasing availability.
These factors contribute to a more inclusive healthcare environment, where individuals can obtain the medications they need without undue financial strain.